This is a long post. So for some, they may want to read it over a day or two.
Remember that recent post I wrote June 1 (https://stroketales.blogspot.com/2019/06/oxygen-getting-to-brain-cells-is.html) about Mike Sheikh and his collaboration with Bioxytran, about the reason brain cells die is all about lack of oxygen in stroke survivors, how clots are really porous?
Mike says, "BXT-25 is like getting hyperbaric oxygen treatment which we know works but only better. The reason it’s better is because it’s the good oxygen that is bound to a molecule not free floating around in the system like the hyperbaric chamber.
"What the hyperbaric chamber does is increase the pressure AND give you 100% oxygen in the process. Normal air only contains 20% oxygen so this means at one atmosphere pressure you get 5 times as much 'free oxygen' floating in the blood. Then at 3 atmospheres at pure oxygen you can get up to 30% more oxygen in your system, but this could put your body under oxidative stress. Too much “free” oxygen is bad for you. Which is why you only get 2 hours in a hyperbaric chamber.
"Contrast versus BXT-25. It’s an injection no 2 hours of cramped conditions and it’s the better type of oxygen therapy that only goes into tissue when needed."
Well, I have new information, brought to you by this person: "He goes by Vision and Value – he likes to remain anonymous," Mike explained. Why anyone wants to remain anonymous with great news escapes me, but here, in essence, is what Vision and Value wrote: (the explanations inside the brackets [ ] are my own because Vision and Value sometimes uses medical terminology, meaning goes cerebral, that you can't possibly know if your outside the health industry).
------
In contrast to breakthroughs in many diseases over the past two decades, stroke treatment, especially from drugs, has had minimal improvement for the past 23 years. Large biopharmaceutical companies such as Biogen are building whole drug development portfolios for stroke, while other companies like SanBio are researching more creative ways to treat stroke, such as via regeneration months after the stroke.
Remember that recent post I wrote June 1 (https://stroketales.blogspot.com/2019/06/oxygen-getting-to-brain-cells-is.html) about Mike Sheikh and his collaboration with Bioxytran, about the reason brain cells die is all about lack of oxygen in stroke survivors, how clots are really porous?
Mike says, "BXT-25 is like getting hyperbaric oxygen treatment which we know works but only better. The reason it’s better is because it’s the good oxygen that is bound to a molecule not free floating around in the system like the hyperbaric chamber.
"What the hyperbaric chamber does is increase the pressure AND give you 100% oxygen in the process. Normal air only contains 20% oxygen so this means at one atmosphere pressure you get 5 times as much 'free oxygen' floating in the blood. Then at 3 atmospheres at pure oxygen you can get up to 30% more oxygen in your system, but this could put your body under oxidative stress. Too much “free” oxygen is bad for you. Which is why you only get 2 hours in a hyperbaric chamber.
"Contrast versus BXT-25. It’s an injection no 2 hours of cramped conditions and it’s the better type of oxygen therapy that only goes into tissue when needed."
Well, I have new information, brought to you by this person: "He goes by Vision and Value – he likes to remain anonymous," Mike explained. Why anyone wants to remain anonymous with great news escapes me, but here, in essence, is what Vision and Value wrote: (the explanations inside the brackets [ ] are my own because Vision and Value sometimes uses medical terminology, meaning goes cerebral, that you can't possibly know if your outside the health industry).
------
In contrast to breakthroughs in many diseases over the past two decades, stroke treatment, especially from drugs, has had minimal improvement for the past 23 years. Large biopharmaceutical companies such as Biogen are building whole drug development portfolios for stroke, while other companies like SanBio are researching more creative ways to treat stroke, such as via regeneration months after the stroke.
However, the fundamental root cause of ischemic injury and the hyperinflammatory after effect is hypoxia [diminished availability of oxygen to the body tissues], a condition that Bioxytran may be able to treat in a very timely manner. Bioxytran Inc. [which] has a novel drug for stroke patients that can restore the supply of oxygen to the brain [even] though clots [exist].
[Here's what you probably know--at least some of it anyway]. Stroke is a leading cause of death in the U.S.; there are approximately 800,000 strokes and 140,000 deaths per year. This accounted for about 1 in 20 deaths between 2000 and 2015. Worldwide, it is the second-largest cause of death. In addition to being the leading cause of disability in the U.S., stroke costs America $34 billion annually in terms of healthcare services, medications and lost productivity. Needless to say, stroke is costly economically.
Some stroke statisticians believe that the cost is underestimated, with annual costs for elderly caregiving alone standing at over $18 billion. The total annual economic cost of stroke may surpass $240 billion by 2030.
There is currently a large unmet medical need for safe and efficacious [effective] therapy, as current treatments are either time-dependent, limited to certain clot types, or both.
Types of Stroke and Current Immediate Post-Stroke Treatments
Stroke is characterized as ischemic (where the issue is a lack of blood supply to a part of the brain), hemorrhagic (blood vessel rupture), or TIA (transient ischemic attack - temporary lack of blood supply).
In the 87% of strokes that are ischemic, a treatment called tPA (tissue plasminogen activator), a clot-breaking agent, is used. Sometimes, a mechanical operation (a thrombectomy) is performed, as well. The problem with tPA is that it has a very limited treatment window, and many people just don’t go to the hospital right away (because they don’t realize they had a stroke). Various studies have shown an absolute maximum treatment window of 4.5 hours. In a recent study, the maximum treatment window was cited at 3 hours, but only ~25% of stroke patients arrived within that time period, and the ideal “door-to-needle” time of less than one hour was only between 11% and 20%. In this study, tPA was administered in 3% of patients in Georgia and 8.5% of patients in Massachusetts. According to the Journal of the American Medical Association (JAMA), the average onset-to-treatment (OTT) time is ~2.5 hours for patients receiving tPA within 4.5 hours of stroke onset.
Thrombectomy
Thrombectomy [surgical removal of blood clot(s)from inside an artery or vein] has a less limited treatment window (6-24 hours), but it is restricted to specific types of clots and is therefore only used in about 9% of patients.
Dire Stroke Outcomes
Despite tPA or thrombectomy therapy, stroke patients will still sustain an injury due to the quick onset of neuronal ischemia and the subsequent inflammation that damages the surrounding penumbra [a space of partial illumination (as in an CT scan) between the perfect shadow on all sides and the full light], where neurons have metabolic activity but significantly decreased electrical activity. For most patients, there is no promise of recovery; whatever cognitive functioning a patient may regain at 90 days post stroke is considered final.
Time Sensitivity of the Ischemic Brain
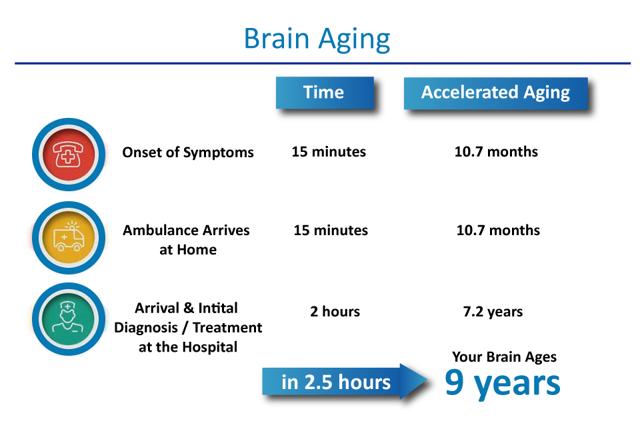
When the supply of blood or oxygen is cut off to the brain for a period of time, brain cells die. Studies have shown that the average ischemic stroke consumes almost 2 million neurons every minute until blood flow is restored. This translates into 14 billion synapses lost and 7.5 miles of myelinated fibers lost every minute, and accelerated aging of the brain by 3.1 weeks. In a typical stroke scenario, 30 minutes is spent getting to the hospital, which results in brain aging of 1.8 years, and then the additional 2 hours to get imaging results in another 7.2 years of aging. This brings the total aging of the brain to 9 years in the average stroke case.
The initial injury, as outlined in my article covering Athersys, is exacerbated by secondary inflammation damage triggered by the initial injury. Thus, minimizing initial injury may help reduce secondary injury via a decrease in damage-associated molecular patterns (DAMPs) and reduced chemokines.
The initial injury, as outlined in my article covering Athersys, is exacerbated by secondary inflammation damage triggered by the initial injury. Thus, minimizing initial injury may help reduce secondary injury via a decrease in damage-associated molecular patterns (DAMPs) and reduced chemokines.
(Source)
Since no treatment exists to get oxygen to the affected parts of the brain, the emphasis had been on awareness and quickly routing patients to stroke treatment centers. As stated before, it is estimated that patients who are able to receive tPA spend 2.5 hours from the onset of stroke until they can get tPA treatment to remove the blockage and restore oxygen supply to the brain. BXT-25, the company’s lead drug, candidate serves as an oxygen bridge for the stroke victim until they can get treatment. This company is addressing a huge unmet medical need that could eliminate a large portion of restorative care and rehabilitation needed after a stroke.
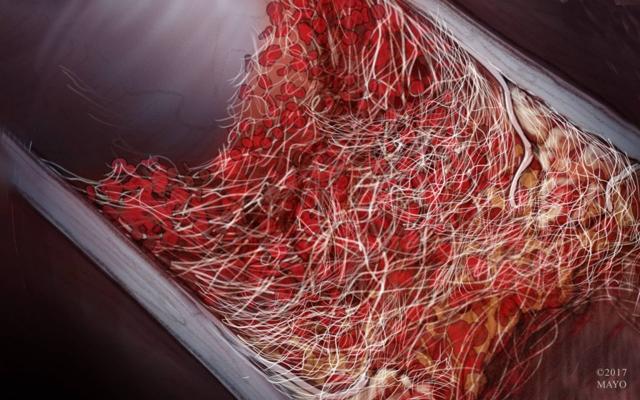
Anatomy of a Thrombus
(Source)
Some may believe that a blood clot is an impermeable barrier; however, it is actually quite porous. Clots that form in the brain’s blood vessels don’t happen overnight either. They are a result of atherosclerosis, which is a build-up of fats and cholesterol on the artery walls. The build-up on the vessel wall constricts the vessel’s cross-section, allowing blood cells to bunch up, which eventually results in fibrin production. Fibrin production is caused by clotting factors and fibrinogen, which is normally dissolved in blood plasma. Through a cascade of interactions, fibrinogen generates thrombin, which converts fibrinogen in the blood plasma into long strands of fibrin that emanate from the clumped cells, designed to trap even more blood cells.
BXT-25: An Elegant Drug Design
Rather than dissolving or breaking up the clot, Bioxytran simply intends to use an oxygen delivery vehicle that is small enough to pass through the clot. Clots act like fishnets, spanning the blood vessel cross-section so anything tiny will pass right through them, but larger items will be caught. BXT-25 is a small drug molecule that is 1/5000th the size of a blood cell and has the ability to easily pass through the clot within the blood plasma.
The Molecule
BXT-25 is a hemoglobin-based polymer that is simply a combination of heme, derived from hemoglobin, and a copolymer designed to stabilize it in the blood system. Heme had been used for years commercially as a meat flavoring, including in the Impossible Burger’s meatless patties. Heme, however, if injected directly into the bloodstream, would be filtered out by the liver.
The ingenious idea conceived by the inventor and CEO of the company, David Platt, was to stabilize the heme and prevent it from being filtered out immediately. He managed to do this with carbohydrate chemistry that mimics natural carbohydrates on blood cells. New blood, created in the bone marrow, has a surface sugar signature that is recognized by the liver. As the blood cell ages, the sugar breaks down to the point where the liver decommissions the cell and filters it out.
BXT-25 is comprised of molecules of heme, which are the oxygen carriers of human blood cells, bonded to a copolymer stabilizer that mimics the sugars found on the blood cells. The polymer is made from the galactoarabinan family of sugar chains and is therefore not metabolized. The most important difference between BXT-25 and blood is that BXT-25 is about 0.02% the size of a red blood cell, and can pass right through a clot and even the blood-brain barrier.
BXT-25 is comprised of molecules of heme, which are the oxygen carriers of human blood cells, bonded to a copolymer stabilizer that mimics the sugars found on the blood cells. The polymer is made from the galactoarabinan family of sugar chains and is therefore not metabolized. The most important difference between BXT-25 and blood is that BXT-25 is about 0.02% the size of a red blood cell, and can pass right through a clot and even the blood-brain barrier.
Other Properties
BXT-25 is specific to O2, as opposed to, say, nitrous oxide (NO). It doesn’t depend on blood to work - it works independently of blood. It is also a neutral sugar in terms of pH - it isn’t alkaline or acidic. The sugar keeps the structure of the heme, and the drug is stable in solution for three years at 250°C.
Oxygen Level Control
One might question whether the oxygen delivered via BXT-25 would be transported specifically to the ischemic portion of the brain after stroke and result in over-oxygenation, which has undesirable side effects. However, this is not likely a problem, because BXT-25 is modeled after the body’s natural control systems, using the same heme as in blood itself, and the company has a means of measuring overall oxygen levels. Many don’t realize that the laws of physics and partial pressure chemistry are responsible for preventing over-oxygenation.
The beautiful thing is that the same principle is true for BXT-25. Since BXT-25 should have a very similar dissociation curve as it uses the same heme from hemoglobin, it can be administered safely to patients and it will properly oxygenate the tissue without over-oxygenating. Furthermore, Bioxytran has access to an FDA-approved device to accurately measure tissue oxygenation so that it can tailor the amount of BXT-25 administered and monitor the tissue metabolism.
Method of Use: First Responders
Once developed and approved, BXT-25 is envisioned to be administered intravenously by first responders to provide oxygen to the brain while they transport the stroke patient via ambulance to a stroke center for further evaluation. The drug could also be administered at the E.R. This is not meant as a long-term solution, but rather as a stop-gap measure to prevent or greatly attenuate the cell death in the brain while the patient is awaiting imaging results, which determines the course of treatment for an ischemic or hemorrhagic stroke.
However, if the patient cannot get a clot removed, it is a possibility that BXT-25 could be used to oxygenate the patient’s brain over a longer period to minimize damage.
(Source)
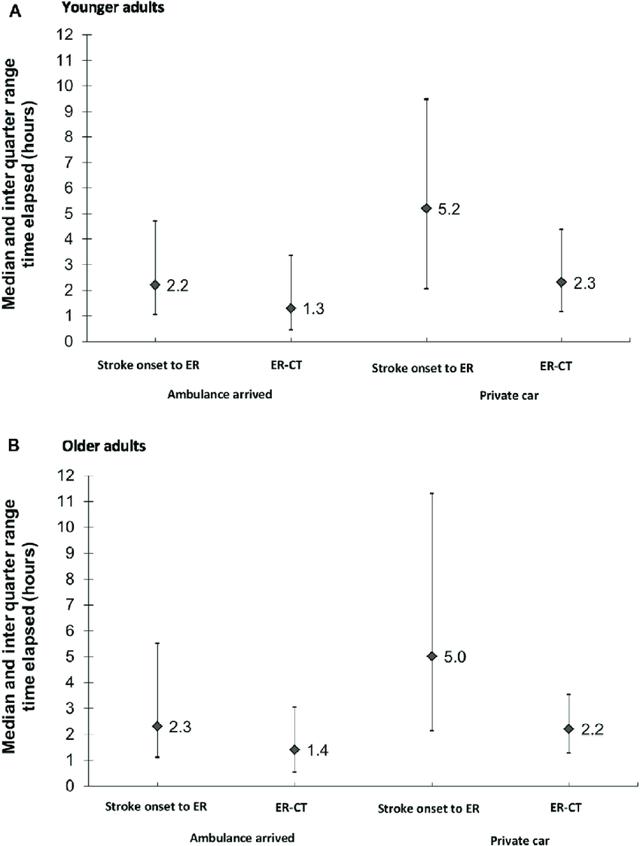
The plot above shows that a typical stroke victim in Israel, even if possibly eligible for tPA or mechanical thrombectomy, may not even arrive at the ER in time to determine whether they do not have a hemorrhagic stroke so that they can get treatment. Many patients are simply too late. As the graph shows, stroke onset to CT time is, on average, 3.7 hours, which is almost past the time possible to be administered tPA. BXT-25 could be administered as soon as the victim is known to have a stroke so that they can have oxygenation much sooner, while they wait for their CT scan and subsequent therapeutic decisions. So, this could be in the ambulance or in the ER.
Chances For Clinical Success
Previous studies have found that even normobaric oxygen therapy within 12 hours can help preserve the aerobic pathway so that the infarct core and penumbra sizes are minimized, as shown by a statistically significant difference in mRS score at discharge after therapy compared to control. How much more effective will permeable, stable heme (BIX-25) be in treating stroke, as it can be administered within the “golden hour” - the critical time for the brain - and physically transport oxygen directly to the injury?
The main takeaway is that BXT-25 is a very simple root-cause solution to stroke. With previous studies of inferior administration of oxygen showing benefit in patients along with the carbohydrate composition of BXT-25, the anticipated chances of success for this drug may be greater than average for other preclinical compounds.
Oxygenation Measurement and Approval Process
The company plans to submit an Investigational New Drug Application (IND) to the Food and Drug Administration (FDA) after completing its master drug file. Preclinical results have shown that the drug is safe and non-toxic. The key to approval rests in Bioxytran’s exclusive license to use the MDXViewer to measure tissue oxygenation. It’s anticipated that tissue oxygenation levels using the Tissue Metabolic Score (TMS) will be measured before the injection and then after. It takes ~3 minutes for BXT-25 to take effect. The TMS is a real-time vital sign of tissue health.
By proving that tissue oxygenation increased, the drug can be approved on that basis alone without making any claim regarding the benefit to stroke patients. That can come in time. When making efficacy claims about benefits to the stroke patients, it’s difficult to quantify which treatment - the BXT-25 or the tPA - ultimately accounted for the benefit. A large, costly trial with multiple arms that uses a subjective impairment study would be the only path forward without the FDA-approved MDXViewer.
By proving that tissue oxygenation increased, the drug can be approved on that basis alone without making any claim regarding the benefit to stroke patients. That can come in time. When making efficacy claims about benefits to the stroke patients, it’s difficult to quantify which treatment - the BXT-25 or the tPA - ultimately accounted for the benefit. A large, costly trial with multiple arms that uses a subjective impairment study would be the only path forward without the FDA-approved MDXViewer.
(Source)
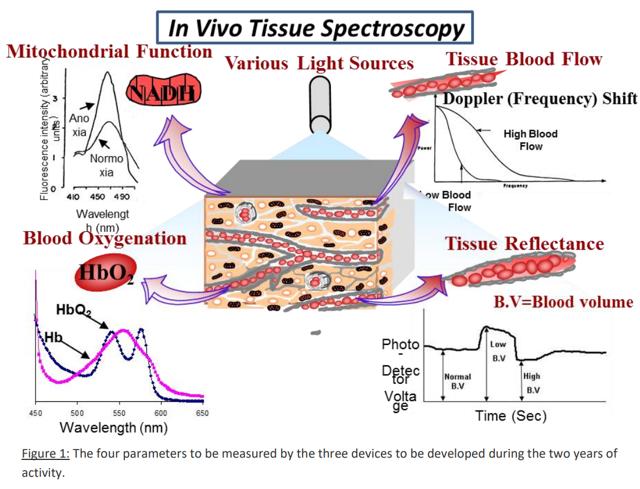
The MDXViewer is a multi-parametric/multivariate monitoring system that measures TMS through four parameters, which are shown in the picture above, through connection to the urethral wall:
- Mitochondrial NADH redox state via surface fluorometry/reflectometry
- Tissue blood flow (TBF, AKA microcirculatory blood flow) via laser Doppler flowmetry
- Blood oxygenation (tissue oxyhemoglobin - HbO2) via spectroscopy
- Tissue blood volume (TBV) via reflectometry
These parameters are then combined with systemic hemodynamic parameters, cardiovascular and respiratory, including heart rate, systemic blood pressure, respiratory rate, systemic hemoglobin saturation and body core temperature. The result is an entire body score, which is important to monitor whenever there is a hypoxic or critical pathologic state in the body.
Essentially, in events including but not limited to perinatal hypoxemia, sepsis, hemorrhagic shock, cardiovascular surgery, heart attack or trauma, the body enters into a body emergency metabolic state (BEMS), where the sympathetic nervous system is activated, adrenaline kicks in and blood flow will be redistributed within the body from all other tissue to the brain, heart and adrenal glands. Thus, the less vital organs will have an energy imbalance and their local mitochondrial production of ATP will be affected.
Essentially, in events including but not limited to perinatal hypoxemia, sepsis, hemorrhagic shock, cardiovascular surgery, heart attack or trauma, the body enters into a body emergency metabolic state (BEMS), where the sympathetic nervous system is activated, adrenaline kicks in and blood flow will be redistributed within the body from all other tissue to the brain, heart and adrenal glands. Thus, the less vital organs will have an energy imbalance and their local mitochondrial production of ATP will be affected.
Future Uses
Stoke is an enormous market, but there are other low-hanging fruit for Bioxytran, such as Acute Respiratory Distress Syndrome (ARDS), which is, in simple terms, lung edema without heart failure. When in an Intensive Care Unit (ICU), many patients develop ARDS, which consists of fluid that leaks into the lungs and eventually develops into a mucus-like substance that forms on and in the lungs’ air sacs (also known as the alveoli). This makes breathing extremely difficult or even impossible; blood cannot move in close enough proximity to the surface of the alveoli to pick up the oxygen.
A small molecule like BXT-25 can go through a much smaller capillary and possibly damaged microvasculature and get closer to the alveoli to bind to oxygen. When the lungs lose their ability to exchange oxygen and carbon dioxide, the body doesn’t get enough oxygen, and eventually, this leads to organ failure and death. The MDXViewer, which measures the tissue oxygenation vital sign, can predict organ shutdown. BXT-25 can provide that lifeline of oxygen to the organs, allowing the body time to recover.
------
We must give thanks to Vision and Value for sharing the great news with us. It's not for just you, I say, but for those who come after and share your gene pool. And Mike Sheikh will stay in touch with me when FDA approval is granted. Stay tuned for this upcoming momentous event!
A small molecule like BXT-25 can go through a much smaller capillary and possibly damaged microvasculature and get closer to the alveoli to bind to oxygen. When the lungs lose their ability to exchange oxygen and carbon dioxide, the body doesn’t get enough oxygen, and eventually, this leads to organ failure and death. The MDXViewer, which measures the tissue oxygenation vital sign, can predict organ shutdown. BXT-25 can provide that lifeline of oxygen to the organs, allowing the body time to recover.
------
We must give thanks to Vision and Value for sharing the great news with us. It's not for just you, I say, but for those who come after and share your gene pool. And Mike Sheikh will stay in touch with me when FDA approval is granted. Stay tuned for this upcoming momentous event!
Very exciting development. I'm looking forward to your updates.
ReplyDeleteDenise, you can count on that!
ReplyDelete